The recent GM massive recall demonstrates how personal injury lawyers play an important role in protecting consumers from defective products and safety hazards
When a Personal Injury Lawyer is representing a plaintiff in a Product Liability case he usually works with a team of experts including engineers who will have to demonstrate that the injuries or the death of the plaintiff were the result of a defective design in the product or a flaw in the manufacturing of the defective product.
In a perfect world manufacturers supervised by regulatory agencies would be making sure that the products they sell are not in any way defective or dangerous to consumers. In reality manufacturers tend to put profits before safety and underfunded regulatory agencies are often too weak to assume their mandate of protecting the consumer.
29 year old Brooke Melton was driving a 2005 General Motors Chevrolet Cobalt when the engine suddenly shut off causing an accident that resulted in her death.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


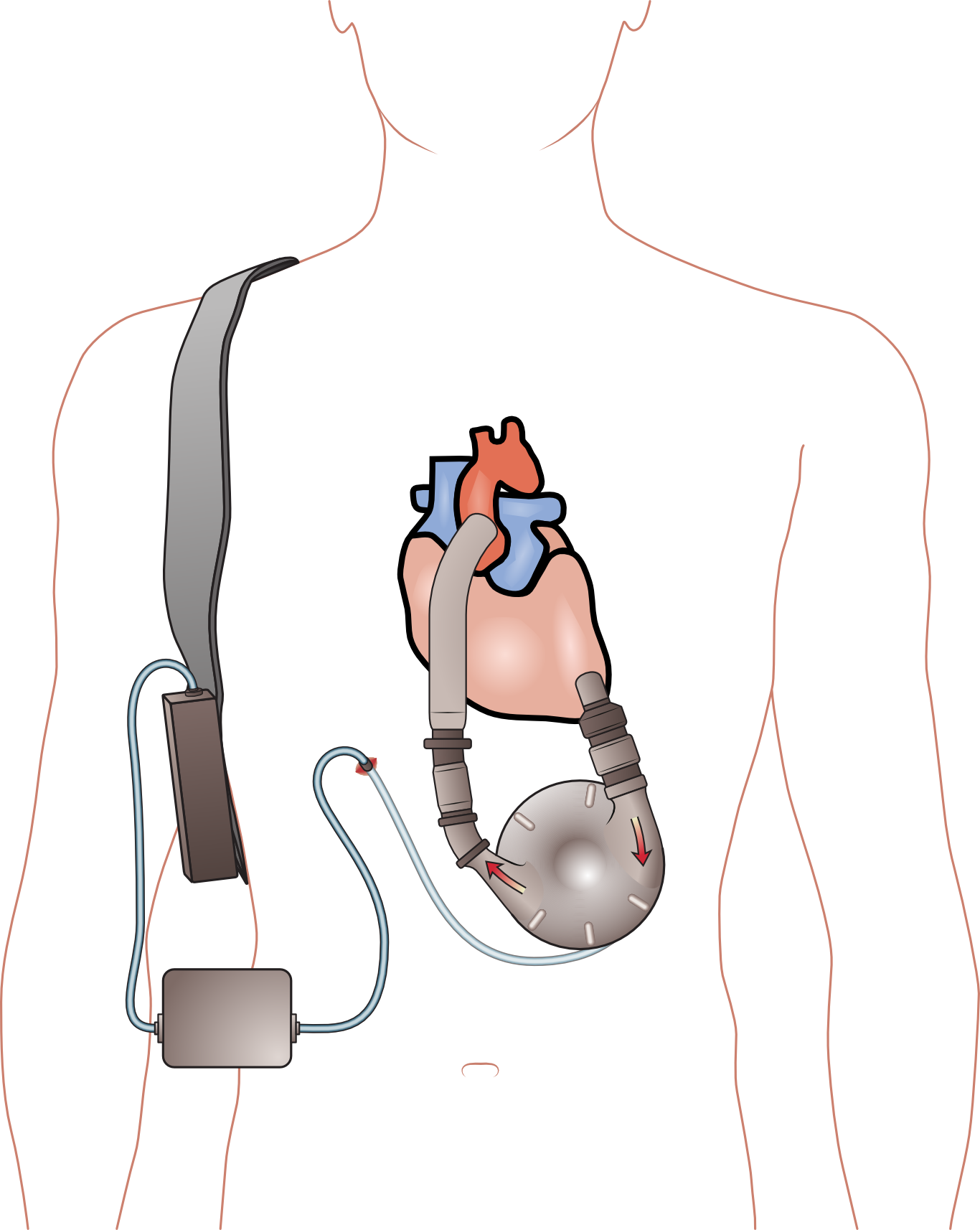 4 patients died and 5 others suffered significant personal injury after Thoratec, a heart device manufacturer launched an updated version of a left ventricular assist device (LVAD), the HeartMate II LVAS, without providing sufficient information and training to patients and caregivers especially those who were originally trained on the older model.
4 patients died and 5 others suffered significant personal injury after Thoratec, a heart device manufacturer launched an updated version of a left ventricular assist device (LVAD), the HeartMate II LVAS, without providing sufficient information and training to patients and caregivers especially those who were originally trained on the older model.