Product Liability: after one child died and another one suffered serious personal injury, Ikea recalls Children’s Wall-Mounted lamp due to strangulation hazard
 A 16 month-old child wrongfully died and a 15 month-old child was severely injured after they became entangled in a very popular Ikea’s children’s wall-mounted lamp.
A 16 month-old child wrongfully died and a 15 month-old child was severely injured after they became entangled in a very popular Ikea’s children’s wall-mounted lamp.
There were 2.9 million lamps sold in the US, 1.1 million in Canada and 23 million worldwide.
Consumers should stop using the defective lamp and contact Ikea at 888 966 4532 for a free repair kit. The repair kit has self-adhesive fasteners for attaching the lamp’s cord to the wall as well as safety instructions.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog



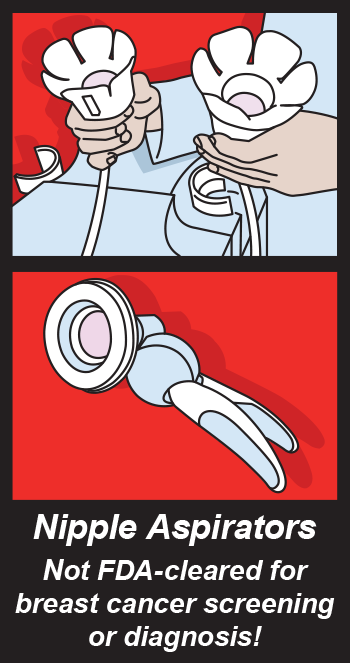 Some companies recently advertised the Nipple Aspirate Test as a method for breast cancer screening. The Nipple AspirateTest consists of using a breast pump to collect fluid from a woman’s nipple to screen for breast cancer. According the the
Some companies recently advertised the Nipple Aspirate Test as a method for breast cancer screening. The Nipple AspirateTest consists of using a breast pump to collect fluid from a woman’s nipple to screen for breast cancer. According the the  Toy testing, toy recalls, toy related injuries and toy shopping will be the subject of the first Google+ Hangout hosted by the CSPC. John Massale, A CSPC engineer, will describe the toy testing process and spokeswoman Nikki Fleming will there to answer all your questions.
Toy testing, toy recalls, toy related injuries and toy shopping will be the subject of the first Google+ Hangout hosted by the CSPC. John Massale, A CSPC engineer, will describe the toy testing process and spokeswoman Nikki Fleming will there to answer all your questions.