Last Friday afternoon 3 pedestrians were seriously injured in a car accident on the Upper East Side. A taxi driver lost control of his vehicle, jumped the curb and plowed into a group of pedestrians on East 86th Street and Third Ave. (see New York Post)
This accident should be fully investigated according to new police regulations recently announced by NYPD Police Commissioner Bratton. In a recent speech Bratton said that not only vehicle accidents leading to death but also vehicle accidents leading to serious personal injury would be fully investigated. (see our previous blog)
On Sunday, a 67 year old pedestrian died in a car accident on Staten Island after being hit by a drunk driver. The pedestrian, James Benedict, was about to enter his car parked on Lily Pond Avenue when he was struck by a 39 year old drunk driver who lost control of his SUV, resulting in the death of Mr. Benedict. The driver was arrested and charged with drunk driving. Read more in silive.com
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


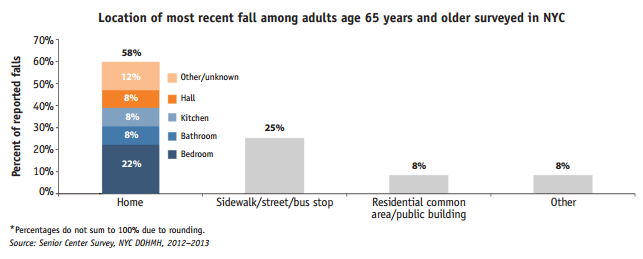 Every year in NYC more than 45,000 seniors suffer personal injury that requires medical treatment after a fall. Around 28,000 of them visit the Emergency Room, 17,000 are hospitalized and 300 die from their injuries. As the New York aging population continues to grow so do the number of personal injuries related to falls. In the report Falls Among Older Adults in New York City, The New York Department of Health indicates that there was an increase of 22% of fall related emergency department visits by seniors between 2006 and 2010.
Every year in NYC more than 45,000 seniors suffer personal injury that requires medical treatment after a fall. Around 28,000 of them visit the Emergency Room, 17,000 are hospitalized and 300 die from their injuries. As the New York aging population continues to grow so do the number of personal injuries related to falls. In the report Falls Among Older Adults in New York City, The New York Department of Health indicates that there was an increase of 22% of fall related emergency department visits by seniors between 2006 and 2010. 
