Knee replacement surgery: robotic versus conventional, what’s best for patients?
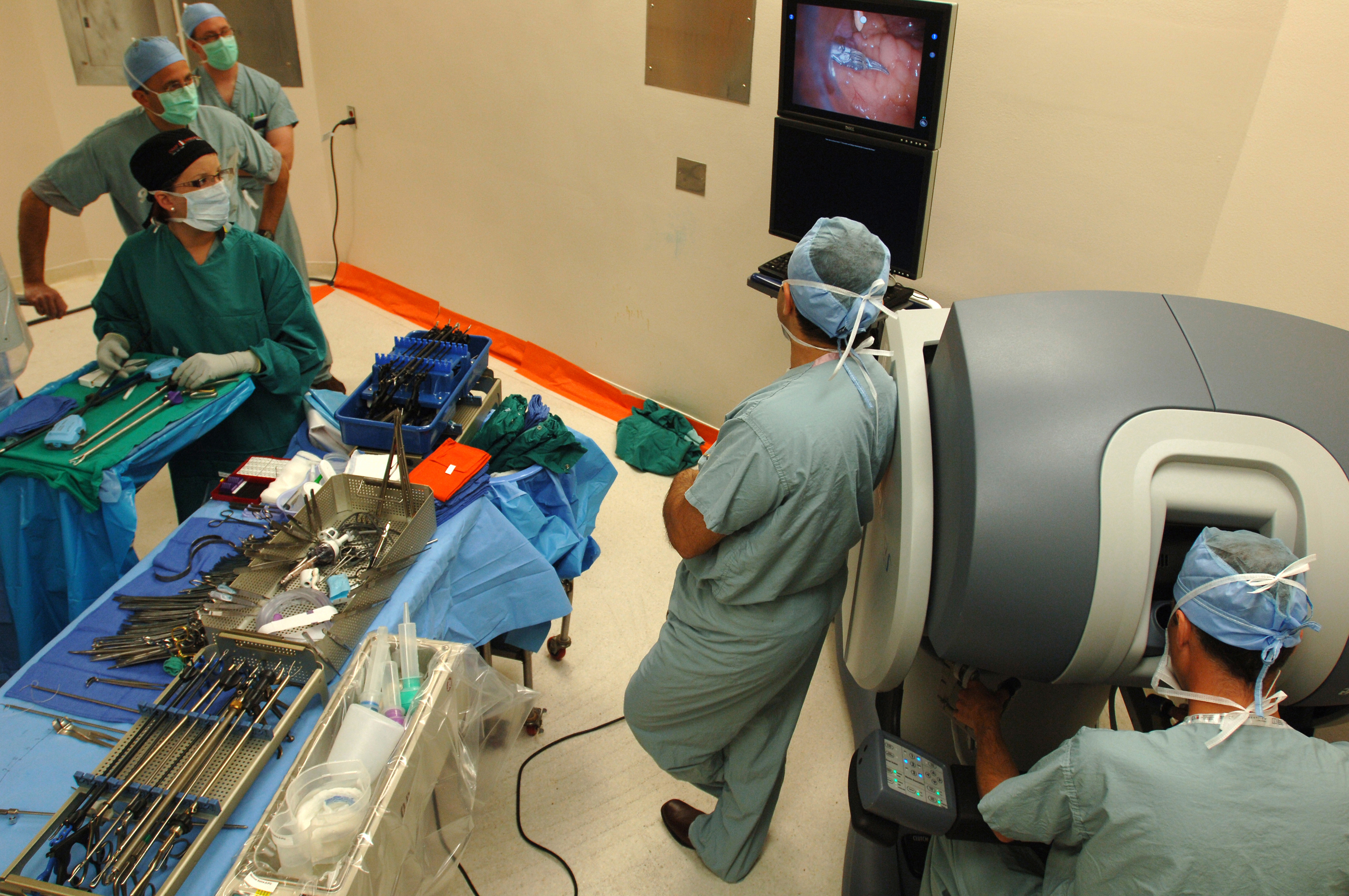
 An investigation presented at the American Academy of Orthopaedic Surgeons meeting in February 2024, delivers a compelling narrative on the comparative outcomes of robotic-assisted knee replacement surgeries versus the conventional approach. As surgical malpractice attorneys entrenched in the intersection of medical innovation and patient outcomes, this study serves as a crucial touchstone for understanding the potential implications on patient care and legal practice.
An investigation presented at the American Academy of Orthopaedic Surgeons meeting in February 2024, delivers a compelling narrative on the comparative outcomes of robotic-assisted knee replacement surgeries versus the conventional approach. As surgical malpractice attorneys entrenched in the intersection of medical innovation and patient outcomes, this study serves as a crucial touchstone for understanding the potential implications on patient care and legal practice.
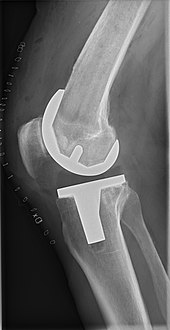
At the heart of the discussion is the study’s revelation: robotic assistance in cementless total knee replacement surgeries does not significantly decrease the likelihood of patients requiring revision surgery within two years when compared to manual methods. This conclusion draws attention not only for its clinical implications but also for its potential to reshape perceptions of medical negligence in the context of emerging surgical technologies.
The research analyzed 9,220 cementless total knee arthroplasty (TKA) procedures recorded in the American Joint Replacement Registry from January 2017 to March 2020. The finding that both robotic-assisted and manual knee replacements had similar rates of implant loosening and infection challenges the narrative that robotic assistance inherently enhances surgical precision and patient outcomes.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog