To prevent medical malpractice and address the risk of spreading CRE infections in hospitals, ECRI institute recommends culturing duodenoscopes
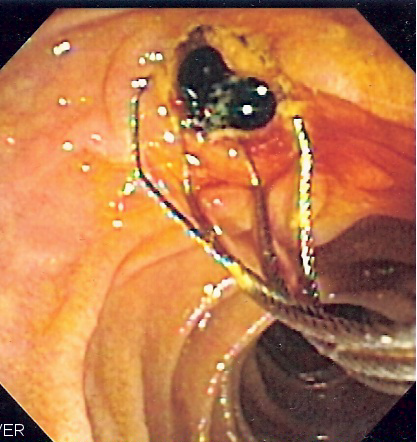 After two patients died and many other suffered personal injury from a recent “superbug”outbreak involving duodenoscopes, the safety of these medical devices (see previous blog) and the method used by hospitals to reprocess them are being questioned. In a recent Hazard Report, the ECRI Institute is recommending culturing Duodenoscopes as a key step to reducing carbapenem-resistant Enterobacteriaceae (CRE). The Institute believes that duodenoscope procedures are vital when treating and diagnosing conditions of the gall bladder and pancreas with Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures and the risk of infection can be mitigated if hospitals upgrade their reprocessing methods by also scope culturing.The Institute recommemds that hospitals not only check with the duodenoscope manufacturer as to whether they are using the appropriate reprocessing method but also add a baseline culture of all duodenoscpoes. Read the complete ECRI High Priority Hazard Report
After two patients died and many other suffered personal injury from a recent “superbug”outbreak involving duodenoscopes, the safety of these medical devices (see previous blog) and the method used by hospitals to reprocess them are being questioned. In a recent Hazard Report, the ECRI Institute is recommending culturing Duodenoscopes as a key step to reducing carbapenem-resistant Enterobacteriaceae (CRE). The Institute believes that duodenoscope procedures are vital when treating and diagnosing conditions of the gall bladder and pancreas with Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures and the risk of infection can be mitigated if hospitals upgrade their reprocessing methods by also scope culturing.The Institute recommemds that hospitals not only check with the duodenoscope manufacturer as to whether they are using the appropriate reprocessing method but also add a baseline culture of all duodenoscpoes. Read the complete ECRI High Priority Hazard Report
Picture Duodenoscopy image of two pigment stones extracted from common bile duct courtesy of Wikipedia
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog


 To prescribe or recommend certain types of pain medicine to a pregnant woman can be medical malpractice that can result in miscarriage, birth defects or attention deficit hyperactivity disorder (ADHD).
To prescribe or recommend certain types of pain medicine to a pregnant woman can be medical malpractice that can result in miscarriage, birth defects or attention deficit hyperactivity disorder (ADHD).  In 2011, the family of 82 year old Pasqualre Vaglio from New York sued a cruise line for medical malpractice after the medical staff on board
In 2011, the family of 82 year old Pasqualre Vaglio from New York sued a cruise line for medical malpractice after the medical staff on board

 Device-related hazards can lead to medical malpractice. In its 2015 top 10 Health Technology hazards, ECRI Institute lists 10 safety topics deemed crucial for hospitals to address. Here is the list of the top 10 technology hazards;
Device-related hazards can lead to medical malpractice. In its 2015 top 10 Health Technology hazards, ECRI Institute lists 10 safety topics deemed crucial for hospitals to address. Here is the list of the top 10 technology hazards; 