Transportation Accidents on Construction Sites Demand Accountability When Construction Workers Are Injured or Killed
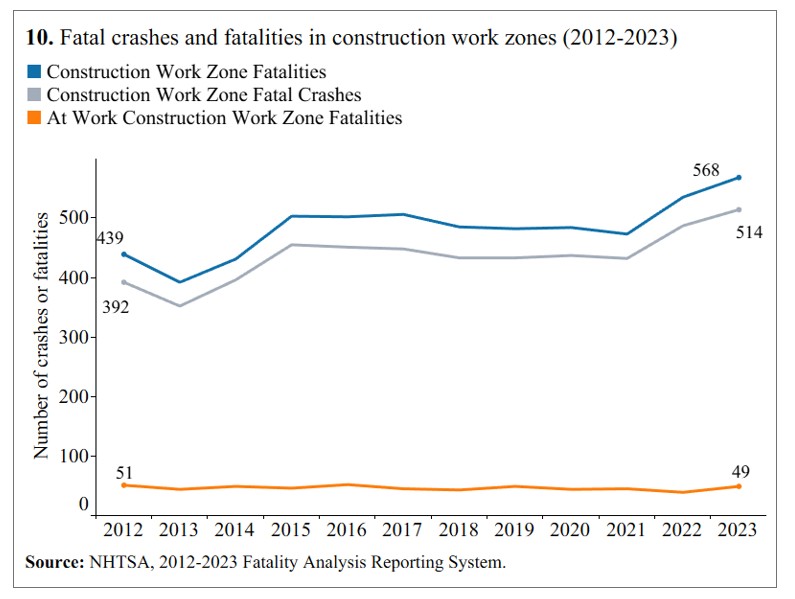 Transportation-related incidents remain one of the most dangerous hazards facing construction workers today. According to data published by The Center for Construction Research and Training, transportation incidents accounted for more than one-third of all occupational fatalities in construction in 2023. These incidents occur both on and off public roadways, often where moving vehicles, heavy equipment, and active work zones intersect. Highway and street construction zones are especially hazardous, placing workers at constant risk of being struck by vehicles or equipment.
Transportation-related incidents remain one of the most dangerous hazards facing construction workers today. According to data published by The Center for Construction Research and Training, transportation incidents accounted for more than one-third of all occupational fatalities in construction in 2023. These incidents occur both on and off public roadways, often where moving vehicles, heavy equipment, and active work zones intersect. Highway and street construction zones are especially hazardous, placing workers at constant risk of being struck by vehicles or equipment.
Nonfatal Transportation Injuries Continue to Rise
Despite improvements in overall safety rates, the total number of nonfatal transportation injuries among construction workers has increased over the past decade. Between 2011–2012 and 2021–2022, nonfatal transportation injuries rose by nearly 15 percent. The majority of these injuries involved roadway incidents with motorized land vehicles, with trucks accounting for the largest share. These injuries frequently occur during routine jobsite activities such as deliveries, equipment movement, and vehicles entering or exiting work zones, where visibility and traffic control are often inadequate.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog