Ethicon Surgical Stapler Recall: FDA Class 1 Alert After One Death and Multiple Adverse Events
 From the Medical Malpractice and Product Liability Attorneys at Gair, Gair, Conason, Rubinowitz, Bloom, Hershenhorn, Steigman & Mackauf
From the Medical Malpractice and Product Liability Attorneys at Gair, Gair, Conason, Rubinowitz, Bloom, Hershenhorn, Steigman & Mackauf
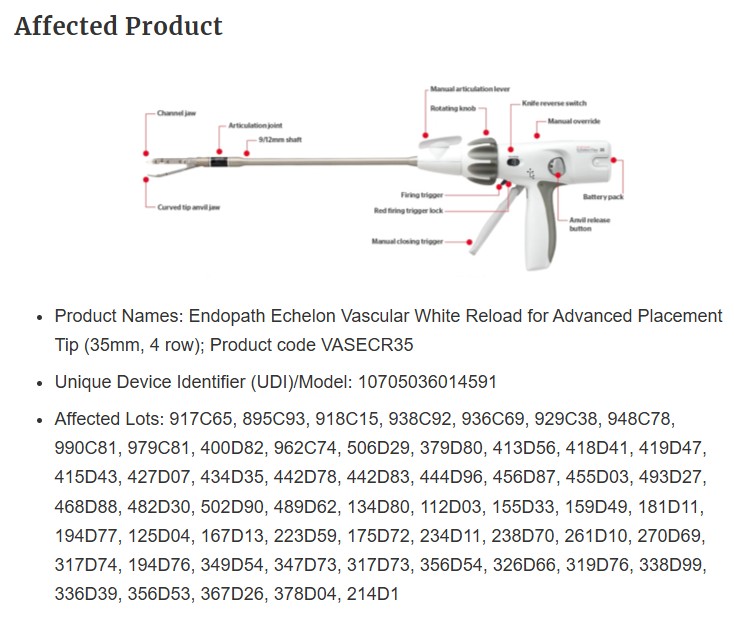
Johnson & Johnson’s Ethicon division has issued a correction and Class 1 recall of its Echelon surgical stapler reload cartridges, following reports of a serious defect linked to at least one death and multiple injuries. The U.S. Food and Drug Administration (FDA) has confirmed that 678,526 units are affected by the recall, which involves staplers used to cut and staple tissue during surgery.
The FDA warned that the stapler may lock during use, failing to properly cut or seal tissue. This malfunction can result in the device becoming trapped in the patient, causing surgical delays, excessive bleeding, hemorrhagic shock, emergency conversion to open surgery, and even death.
 New York Personal Injury Attorneys Blog
New York Personal Injury Attorneys Blog